Abstract

Background: Splenectomy is an effective therapy for chronic immune thrombocytopenia (ITP), with high rates of long-term responses reported (about 60-80%). Because the possible related complications its indication has been limited, proving the need for further research into potential predictive factors of response. The platelet clearance determined by 111In-labelled autologous platelet scintigraphy has been proposed as an independent predictor of splenectomy outcome, with heterogenous recorded results.This study aimed to evaluate the effect of splenic platelet sequestration in predicting splenectomy response, as well as to identify other possible factors that could justify treatment failure.
Methods: We retrospectively analyzed a cohort of refractory and relapsed ITP patients who underwent 111In-labelled platelet scintigraphy in one center in Madrid (La Paz University Hospital), between 2005 and 2021. The sequestration patterns were categorized based on the splenic:liver ratio (S:L), and dichotomized as splenic if purely splenic (S:L > 2) or predominantly splenic (S:L 1.4 < 2); and non-splenic if mixed (S:L 0.8 < 1.4) or hepatic (S:L < 0.8). The response to splenectomy was assessed in terms of blood platelet count at 3, 6 and 12 months post-splenectomy, according to ITP Spanish Group 2020 guidelines. Biological platelet characteristics were subsequently examined to investigate their role in splenectomy failure. Platelet activation markers (such as activation of fibrinogen receptor determined through PAC1-binding, TRAP-induced P-selectin and CD63 exposure) and active caspase-3, -7, -8 or -9 were evaluated. Platelet surface sialic acid exposure was studied by determining the binding of Ricinus Communis Agglutinin (RCA) labelled with fluorescein (FITC). Samples were analyzed by flow cytometry. Results were expressed as the ratio between each patient's values and the mean from healthy control group. Patients were separated into responders and non-responders for the analysis, applying univariate tests and multivariate regression models.
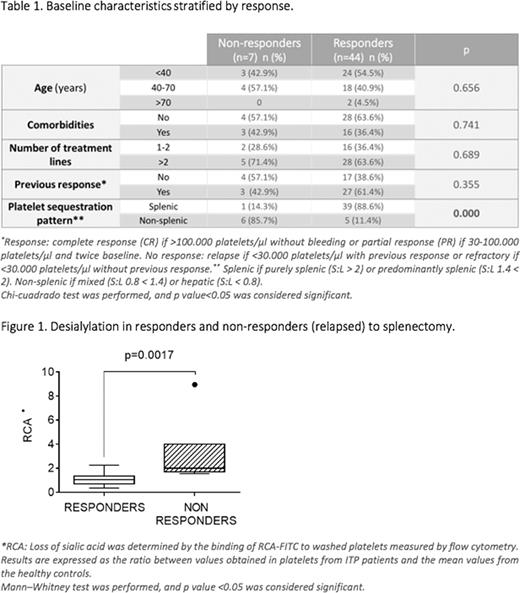
Results: Platelet scintigraphy was performed in 80 patients, 51 of them underwent splenectomy. Median previous lines of treatment were 3.5 (2-6). Complications occurred in 10% of splenectomized patients, with only one non-hemorrhagic fatality event in the cohort (not related to splenectomy). Among the 51 patients with splenectomy performed, 86.2% did respond. When analyzed by sequestration pattern, complete response (CR) was observed in 75% of those with a splenic pattern. By univariate analysis, only splenic sequestration correlated with higher sustained response rate at 12 months (Table 1). The same parameter retained statistical significance through a multivariate logistic regression model (Odds ratio=37.65; p=0.005). No association was observed in terms of response with the other analyzed factors, including age, comorbidity, previous lines of treatment and previous response. Seven responders patients with splenic clearance relapsed within 3 months. Their platelet characteristics were compared to those from 16 responders controls. There were no significant differences between groups in the variables studied, except for increased binding of RCA to platelets of patients who relapsed (median, percentile 25/75= 2.05, 1.74/4.03), in contrast to those who maintained response (1.05, 0.71/1.37) (Figure 1). These findings suggest an increased desialylation pattern in platelets from the patients who relapsed.
Conclusions: Our data confirm the predictive value of 111In-labelled platelet studies prior to splenectomy, with splenic sequestration emerging as an independent predictor of long-term response. Enhanced desialylation in platelets surface provides a potential explanation for splenectomy failure in those patients with a splenic clearance. The clinical relevance of these results is due to enabling a better selection of suitable candidates for splenectomy, to increase its efficacy and safety.
Keywords: Chronic Immune Thrombocytopenia (ITP), splenectomy, autologous 111In-labelled platelet, splenic sequestration.
Study supported by ISCIII-Fondos FEDER (PI19/00772) and PDSA.
Disclosures
Butta:Takeda: Research Funding, Speakers Bureau; Novo-Nordisk: Speakers Bureau; Roche: Speakers Bureau; CSL-Bering: Research Funding. Ramirez Lopez:Janssen: Speakers Bureau; Sanofi: Speakers Bureau; Kyowa: Speakers Bureau. Hermans:oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Honoraria; oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Research Funding; oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, BioMarin, Octapharma, CSL Behring: Consultancy. Jiménez-Yuste:Roche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, BioMarin, Octapharma, CSL Behring: Consultancy; Roche, NovoNordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Honoraria; Roche, NovoNordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Octapharma, CSL Behring: Research Funding. Alvarez Román:Biomarin: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board ; CSL-Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Octapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.